These notes teach you about chemical reactions processes that form new substances through atom rearrangement, with everyday examples like milk curdling, rusting, and burning .You will learn to identify reactants and products, write word/formula equations, and balance them using the hit-and-trial method to follow the Law of Conservation of Mass.
1. Chemical Changes and Chemical Reactions
- Chemical Change: A process where new substances form with different properties (irreversible).
- Examples:
- Milk → Curd (adding lemon juice): Milk turns thick and sour due to coagulation of casein protein.
- Rusting of iron: Iron reacts with oxygen (and moisture) to form rust (iron(III) oxide).
- Burning of firewood: Wood (mainly cellulose) burns to form ash, carbon dioxide, and water vapor.
- During chemical reactions: Atoms rearrange through combination, decomposition, or exchange.
- Chemical Reaction: Process involving exchange, combination, or decomposition of atoms.
2. Reactants and Products
- Reactants: Substances that take part in the reaction (left side).
- Products: New substances formed (right side).
- Example: Carbon + Oxygen → Carbon dioxide Reactants: Carbon, Oxygen Product: Carbon dioxide
3. Chemical Equation
- Representation of a chemical reaction using symbols/formulae.
- Types:
- Word Equation: Uses words (e.g., Zinc + Hydrochloric acid → Zinc chloride + Hydrogen gas).
- Formula Equation: Uses symbols/formulae (e.g., Zn + 2HCl → ZnCl₂ + H₂).
4. Balanced vs Unbalanced Chemical Equation
- Unbalanced: Number of atoms of each element unequal on both sides.
- Balanced: Equal number of atoms of each element on both sides (follows Law of Conservation of Mass).
- States: (s) solid, (l) liquid, (g) gas, (aq) aqueous.
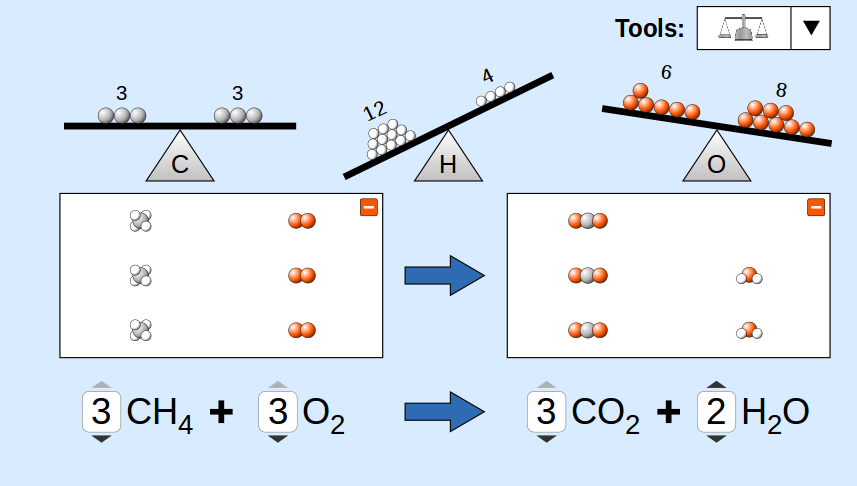

5. Steps to Balance (Hit and Trial Method)
- Write word equation.
- Write formulae of reactants and products.
- Do not change subscripts in formulae.
- Add coefficients to balance atoms (start with most complex molecule).
- Count atoms on both sides to verify.
Key Examples of Balanced Equations:
- Hydrogen + Oxygen → Water 2H₂ + O₂ → 2H₂O
- Zinc + Hydrochloric acid → Zinc chloride + Hydrogen Zn + 2HCl → ZnCl₂ + H₂

- Magnesium + Oxygen → Magnesium oxide 2Mg + O₂ → 2MgO

- Calcium carbonate (heat) → Calcium oxide + Carbon dioxide CaCO₃ → CaO + CO₂ (already balanced)
- Rusting of iron: 4Fe + 3O₂ → 2Fe₂O₃
The Method of Writing a Balanced Chemical Equation
A balanced chemical equation has an equal number of atoms of each element on both the reactants (left) and products (right) sides. This follows the Law of Conservation of Mass, which states that mass (or atoms) cannot be created or destroyed in a chemical reaction.


The most common method for Grade 9 level is the Hit-and-Trial Method (also called Trial-and-Error Method).
Steps in Hit-and-Trial Method:
- Write the word equation first.
- Write the correct molecular formulae of reactants and products to make a skeleton (unbalanced) equation.
- Do not change subscripts in the formulae (e.g., don't change H₂O to H₄O).
- Balance atoms by adding coefficients (numbers in front) to molecules. Start with the most complex molecule or the element that appears in fewer compounds.
- Balance one element at a time (usually metals first, then non-metals, then H and O last).
- Count the number of atoms on both sides and repeat until all are equal.
- Optionally, add state symbols: (s) solid, (l) liquid, (g) gas, (aq) aqueous.


Example 1: Hydrogen + Oxygen → Water (Classic from your textbook)
- Skeleton: H₂ + O₂ → H₂O (Unbalanced: H = 4 on right? Wait—no: left H=4? No.)
Actually:
- Unbalanced: H₂ + O₂ → H₂O Atoms: Left - H:2, O:2 | Right - H:2, O:1 → Unbalanced.
- Step: Balance O by putting 2 in front of H₂O → H₂ + O₂ → 2H₂O Now: Left - H:2, O:2 | Right - H:4, O:2 → H unbalanced.
- Balance H by putting 2 in front of H₂ → 2H₂ + O₂ → 2H₂O Now balanced: H:4 each side, O:2 each side.


Example 2: Magnesium + Oxygen → Magnesium Oxide
- Skeleton: Mg + O₂ → MgO Unbalanced (Mg:1=1, O:2=1).
- Balance O: Mg + O₂ → 2MgO (now O:2=2, Mg:1=2).
- Balance Mg: 2Mg + O₂ → 2MgO (balanced).

How to Balance a Chemical Equations? - SciBond
Unbalanced vs Balanced Illustration
Unbalanced equations have unequal atoms; balanced ones are equal.


Tip for Exams: Always count atoms after each step. Practice with equations like Zn + HCl, Fe + O₂ (rusting), etc. The equation must obey conservation of mass—no atoms lost or gained!
6. Information from Balanced Equation
- Names and formulae of reactants/products.
- Number of molecules/atoms.
- Ratio of masses.
- Type of reaction.
7. Importance of Chemical Reactions
- Digestion of food.
- Respiration (energy production).
- Photosynthesis.
- Combustion of fuels.
- Curdling of milk, medicines working, etc.