Classification of Elements
Around us, matter exists as elements, compounds, and mixtures.
There are 118 elements, and they do not all have the same type of atoms and not the same properties.
So to study so many elements easily, scientists classified them.
What is Classification of Elements? (Definition)
Classification of elements means arranging all the known elements in a systematic manner based on their similar chemical and physical properties.
This arrangement helps us study, predict, and understand the behavior of elements easily.
Why classify elements?
Because:
Elements have different physical and chemical properties.
Some elements have similar properties.
Classification helps understanding, predicting behavior, and studying elements easily.
2. Historical Developments
(a) Döbereiner’s Triads (1829)
Elements arranged in groups of three with similar properties.
Atomic mass of the middle element ≈ average of other two.
Example:
Li (7), Na (23), K (39)
23 ≈ (7 + 39) / 2
Limitations:
Worked only for a few triads.
(b) Newlands’ Law of Octaves (1864)
When arranged by increasing atomic mass,
every 8th element had properties similar to the 1st.
Limitations:
Worked only up to Calcium.
Failed for heavier elements.
Transition metals did not fit.
(c) Lothar Meyer’s Curve (1869)
Plotted atomic volume vs atomic mass.
Elements at peaks → alkali metals.
Demonstrated periodicity.
(d) Mendeleev’s Periodic Table (1869)
Arranged elements by increasing atomic mass.
Achievements:
Left gaps for undiscovered elements.
Predicted properties of Ga, Ge, Sc accurately.
Grouped elements correctly.
Limitations:
Increasing atomic mass did not always work (e.g., Co & Ni).
Position of hydrogen unclear.
Isotopes did not fit.
3. Modern Periodic Law
“Properties of elements are a periodic function of their atomic number.Because atomic number (Z) is the real basis of periodicity.
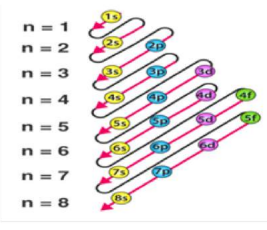
4. Modern Periodic Table Features
Periods (Horizontal Rows): 7
Period number = number of shells (n).
Groups (Vertical Columns): 18
Group number = number of valence electrons (except transition metals).
Blocks
s-block → Groups 1–2
p-block → Groups 13–18
d-block → Groups 3–12 (transition metals)
f-block → Lanthanides & actinides
s-block: Group 1–2
Highly reactive metals
Form basic oxides
p-block: Group 13–18
Contains metals, non-metals, and metalloids
Includes halogens and noble gases
d-block: Group 3–12
Transition metals
Show color, variable oxidation state
f-block: Lanthanides & Actinides
Inner transition elements
Mostly radioactive (actinides)
6. Key Differences (Exam Favorites)
Metals vs Non-metals
MetalsNon-metalsShinyDullGood conductorsPoor conductorsForm cationsForm anionsMalleableBrittle
s-block vs p-block
s-blockp-blockGroups 1–2Groups 13–18Highly reactiveMixed types of elementsSimple electronic configComplex behavior
7. PERIODIC PROPERTIES (Most Important Part)
(A) Atomic Radius (Size of atom)
Across a period → decreases
(more nuclear charge pulls electrons closer)
Down a group → increases
(more shells added)
(B) Ionic Radius
Cations (positive ions) → smaller (lose electrons)
Anions (negative ions) → larger (gain electrons)
(C) Ionization Energy (IE)
Energy to remove one electron.
Across a period → increases
Down a group → decreases
Low IE = Alkali metals
High IE = Noble gases
(D) Electron Affinity (EA)
Energy released when an atom gains an electron.
Across period → increases
Down group → decreases
Highest EA = Chlorine (NOT Fluorine)
(E) Electronegativity (EN)
Tendency to attract shared electrons.
Across period → increases
Down group → decreases
Most EN element = Fluorine
(F) Metallic Character
Decreases from left to right
Increases from top to bottom
Opposite to non-metallic character.
8. Special Cases (Anomalies)
Very important for board exams.
Hydrogen behaves like alkali metals and halogens → special position
Helium belongs to noble gases though it is s-block
Lanthanide contraction affects d-block sizes
Co & Ni atomic mass issue fixed by modern law
9. Valency
Valency is the number of electrons an atom gains, loses, or shares.
s-block: Valency = group number
p-block: varies (commonly 3, 4, 5, etc.)
10. Why Periodicity Occurs?
Because elements have repeating electronic configurations at regular intervals.
Example:
Group 1: ns¹
Group 2: ns²
Group 17: ns² np⁵
Gallery

Henry Moseley and Periodic Table
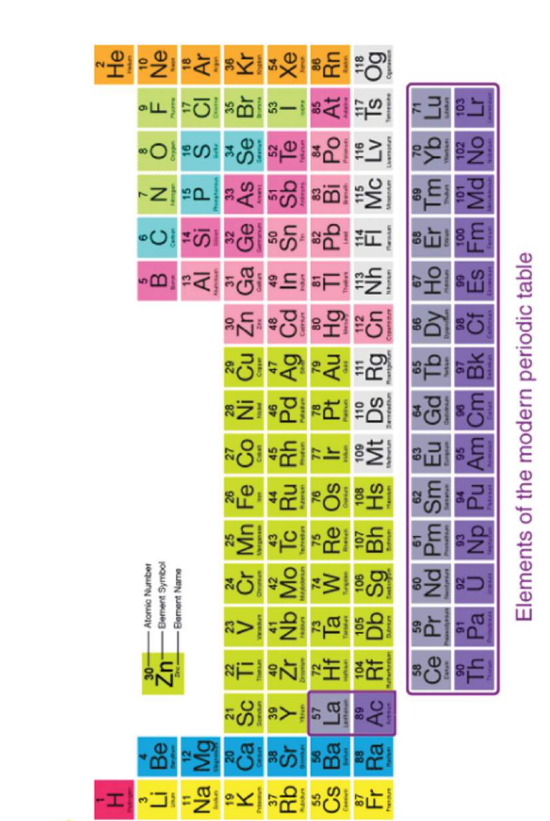
Modern periodic Table