1. Introduction to Heat
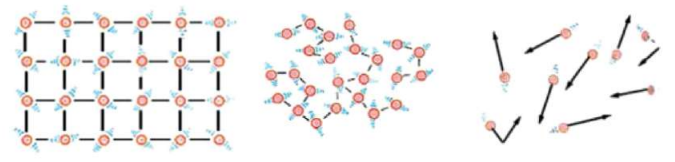
All matter around us is made of tiny particles called atoms or molecules. These particles are always moving. When they move faster, the substance becomes hotter; when they move slower, it becomes colder.
Heat is a form of energy transferred from a hotter object to a colder object because of a difference in temperature.
It is measured in joules (J).
Heat is never stored inside an object. What is stored is thermal energy, which is the total kinetic energy of the particles in the object.
2. Thermal Energy
Thermal energy depends on two things:
Mass of the object
Average kinetic energy (speed) of the molecules
Hotter molecules move fast and have more kinetic energy. Colder ones move slow.
Example:
A pan of boiling water (100°C) has higher temperature, but a bucket of lukewarm water (30°C) may have more thermal energy because it contains more water molecules.
3. Temperature
Temperature measures average kinetic energy of the particles.
SI unit: Kelvin (K)
Common unit: Celsius (°C)
Important:
0 K = -273.15°C (absolute zero, where molecular motion is theoretically zero).
4. Heat Transfer
Heat is transferred through:
Conduction
Convection
Radiation
(A) Conduction
Transfer of heat through solids without movement of particles.
Example:
A steel spoon becomes hot when placed in hot soup because heat travels through metal.
(B) Convection
Transfer of heat in liquids and gases through actual movement of particles.
Example:
Warm air rises and cool air sinks, forming currents.
(C) Radiation
Transfer of heat through empty space in the form of electromagnetic waves.
Example:
We feel the heat of the Sun even though space has no air.
5. Scientific Reasons Behind Precautions (VERY IMPORTANT)
PrecautionMethodScientific ReasonWe should not put our hands over boiling waterConvectionSteam molecules move very fast and carry lots of energy. They penetrate skin easily and cause severe burns.Do not pour hot water into a glass tumbler in winterConductionInner surface expands quickly but outer surface stays cold; uneven expansion creates stress and the glass cracksDo not keep a completely full water bottle in the deep freezerExpansionWater expands when freezing; increased volume creates pressure and the bottle cracks.Do not pick a steel plate covering hot food with bare handsConductionMetals conduct heat quickly. Heat travels instantly from plate to hand and burns the skin.6. Effect of Heat on Volume (Expansion)
All states of matter expand when heated because particles vibrate faster and move apart.
Solids:
Expand the least because particles are tightly packed.
Liquids:
Expand more than solids.
Gases:
Expand the most because particles are far apart and move freely.
7. Activity: Iron Ball and Ring (Expansion)
When the iron sphere is heated, molecules vibrate faster, move apart, and the sphere expands → does not pass through the ring.
When cooled, molecules slow down and come closer → sphere contracts → fits through the ring again.
8. Anomalous Expansion of Water (VERY IMPORTANT)
Water behaves differently from most substances.
From 0°C to 4°C
Water contracts (volume decreases).
Density increases and becomes maximum at 4°C.
From 4°C to 0°C
Water expands (volume increases).
Ice at 0°C is less dense and floats.
Effects:
In ponds and lakes, water at 4°C sinks to the bottom. Water above freezes at the top. Aquatic animals survive below the ice.
Water pipes burst in winter because water expands on freezing.
Bottles filled with water crack in freezers.
9. Specific Heat Capacity
Specific heat capacity (s) =
Heat required to raise the temperature of 1 kg of a substance by 1°C
Unit: J/kg°C
Heat equation:
Q = ms(T₂ – T₁)
Where Q = heat gained/lost
m = mass
s = specific heat capacity
Substances and Their Specific Heat Capacities
Water: 4200 J/kg°C
Ice: 2100 J/kg°C
Aluminum: 884 J/kg°C
Iron: 460 J/kg°C
(And others…)
Why Water Has High Specific Heat Capacity (Uses)
Because water needs a large amount of heat to increase its temperature:
Uses:
Water is used as a coolant in car radiators and power plants.
- Hot water bags stay warm for long.
- Wet cloth cools a fever patient slowly.
- Coastal areas have smaller temperature differences between day and night.
- Sea breeze and land breeze occur.
10. Sea Breeze and Land Breeze
Sea Breeze (Daytime)
- Land heats faster than sea.
- Warm air above land rises.
- Cool air from sea moves toward land.
Land Breeze (Night)
- Land cools faster than sea.
- Warm air above sea rises.
- Cool air from land moves toward sea.
11. Measurement of Temperature
1. Liquid-in-Glass Thermometer
Contains mercury or alcohol.
When heated, liquid expands and rises.
Scale shows the temperature.
2. Digital Thermometer
Uses a thermistor.
Change in resistance → change in temperature shown on display.
3. Infrared (Radiation) Thermometer
Measures infrared radiation emitted by object.
No contact needed.
12. Calibration of Thermometer
Calibration means marking the scale correctly.
Steps:
- Put bulb in melting ice → mark 0°C (lower fixed point).
- Put bulb in steam from boiling water → mark 100°C (upper fixed point).
- Divide the distance into 100 equal parts → each part = 1°C.
Different scales use different fixed points:
- Celsius: 0°C to 100°C
- Fahrenheit: 32°F to 212°F
- Kelvin: 273 K to 373 K
13. Heat Equation (Summary)
Q = msΔT
s = Q / (mΔT)
Greater mass → more heat needed
Greater temperature difference → more heat needed
COMPLETE SUMMARY FOR QUICK REVISION
- Heat = energy transferred due to temperature difference.
- Temperature = average kinetic energy of particles.
- Thermal energy = total kinetic energy of particles.
- Heat transfer methods: conduction, convection, radiation.
- Expansion: solids < liquids < gases.
- Water shows anomalous expansion.
- Specific heat capacity determines how fast something heats up.
- Water has high SHC → used as coolant.
- Sea breeze (day), land breeze (night).
- Thermometers: liquid-in-glass, digital, radiation.
- Calibration uses fixed points: 0°C and 100°C.
Gallery
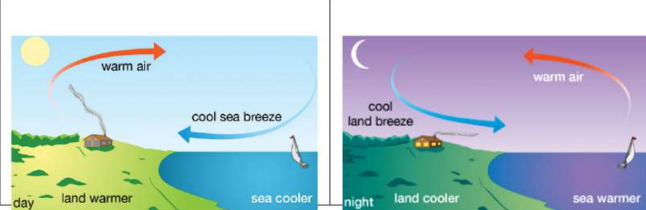
See Breeze
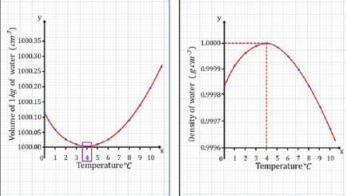
Relation between the volume and density of water, and between the density and temperature of the water